

Stilla Technologies Digital PCR System
naica®Crystal Digital PCR System
NaicaTMCrystal Digital PCR System
Azure Biosystems Real-Time PCR Systems

Malaria is caused by unicellular protozoan parasites of the Plasmodium genus,The eradication of human malaria would save more than 400,000 lives annually and prevent approximately 200 million cases of the disease each year.
The eukaryotic cell cycle is typically divided into distinct phases with cytokinesis immediately following mitosis. To ensure proper cell division, each phase is tightly coordinated via feedback controls named checkpoints. During its asexual replication cycle, the malaria parasite Plasmodium falciparum undergoes multiple asynchronous rounds of mitosis with segregation of uncondensed chromosomes followed by nuclear division with intact nuclear envelope. The multi-nucleated schizont is then subjected to a single round of cytokinesis that produces dozens of daughter cells called merozoites. To date, no cell cycle checkpoints have been identified that regulate the Plasmodium spp. mode of division. Given the peculiarity of cell division in Plasmodium parasites, understanding the molecular mechanisms that drive and regulate these processes in the parasite could unveil novel targets for the treatment of malaria. The recommended article " Depletion of the mini-chromosome maintenance complex binding protein allows the progression of cytokinesis despite abnormal karyokinesis during the asexual development of Plasmodium falciparum " found a protein complex related to the division of Plasmodium falciparum. Causes abnormal cell division of malaria parasites.

The author identify the Plasmodium homolog of the Mini-Chromosome Maintenance Complex Binding Protein (PfMCMBP), which co-purified with the MCM complex, a replicative helicase required for genomic DNA replication. To investigate the role of PfMCMBP during the asexual life cycle of P. falciparum parasites, The author fused three copies of the hemagglutinin epitope (HA) tag with a destabilization domain (DD) to the carboxy-terminus of the endogenous PfMCMBP in the 3D7 strain by homologous recombination, generating the 3D7-PfMCMBP3HADD transgenic parasite line. In the presence of the small molecule Shield-1 (Shld1), PfMCMBP3HADD protein is stabilized, while in absence of Shld1, the protein is rapidly degraded. By Echo fluorescence microscopy, depletion of PfMCMBP disrupt nuclear morphology and parasite proliferation without causing a block in DNA replication. PfMCMBP depletion promotes the formation of MTOCs with extended spindle microtubules that are attached to chromosomes in spatially separated clusters of DNA.
PfMCMBP depletion promotes the formation of mitotic spindle microtubules with extensions to more than one DNA focus and abnormal centrin distribution. Strikingly, PfMCMBP-deficient parasites complete cytokinesis and form aneuploid merozoites with variable cellular and nuclear sizes.The study demonstrates that the parasite lacks a robust checkpoint response to prevent cytokinesis following aberrant karyokinesis.
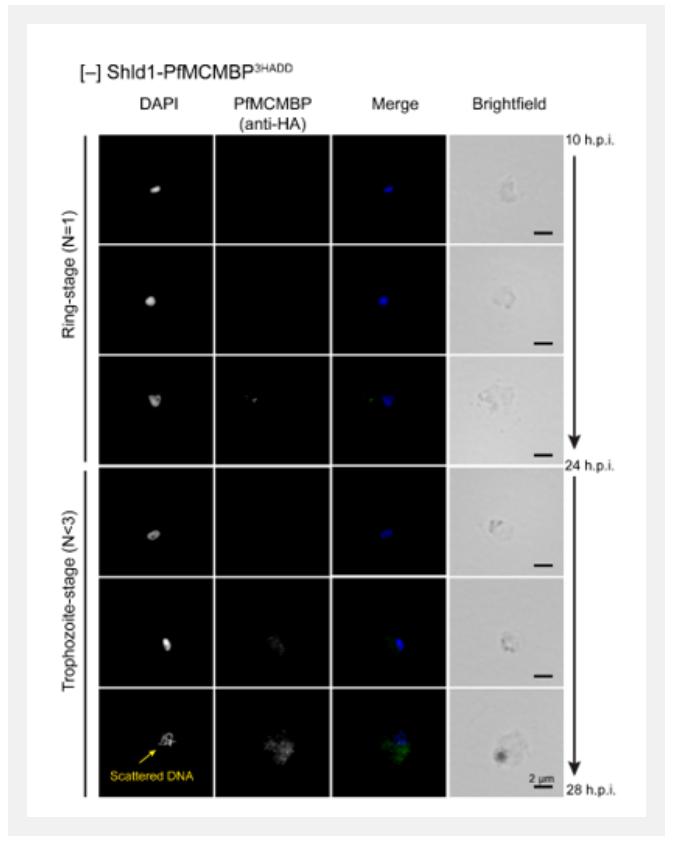
▲ Nuclear DNA shape of PfMCMBP-deficient parasites throughout ring-stage up to early-trophozoite-stage.
Echo Hybrid Microscope

▲ Echo Hybrid Microscope
Achieve extraordinary performance:
Sajuthi S P , Deford P , Li Y C , et al. Type 2 and interferon inflammation regulate SARS-CoV-2 entry factor expression in the airway epithelium[J]. Nature Communications, 2020, 11(1).DOI:10.1038/s41467-020-18781-2
