

Stilla Technologies Digital PCR System
naica®Crystal Digital PCR System
NaicaTMCrystal Digital PCR System
Azure Biosystems Real-Time PCR Systems

The sudden outbreak of COVID-19 epidemic in 2019 still profoundly affects our lives and society today, and the prevention of the new crown virus SARS-CoV-2 has become a part of our daily lives,Researchers continue to explore the treatment, protection, immunity and other aspects of the new coronavirus to help us understand the virus and overcome the epidemic. Recently, a report from the Jewish Health Center in the United States helped us better understand the pathogenesis of the new coronavirus. The article was published in "Nature commuunications", revealing the possible mechanism that affects the infectiousness of SARS-CoV-2 and the clinical outcome of new coronary pneumonia .

Coronavirus disease 2019 (COVID-19) is caused by SARS-CoV-2,Studies have found that the expression of host proteins ACE2 and TMPRSS2 in the respiratory tract is related to the patient's infection of new coronary pneumonia,SARS-CoV-2 can use host proteins ACE2 and TMPRSS2 as entry factors to invade the human body。The researchers explore the role of genetics and co-expression networks in regulating these genes in the airway, through the analysis of nasal airway transcriptome data from 695 children. The researchers identify expression quantitative trait loci for both ACE2 and TMPRSS2, that vary in frequency across world populations,For example,the TMPRSS2 eQTL variant associated with decreased expression,rs1475908, occurred across all world populations, with the highest allele frequencies among East Asians (AF = 38%), Europeans (AF = 35%), intermediate frequencies among Africans (AF =26%) and Ashkenazi Jews (AF = 23%), and the lowest frequency among Latinos (AF = 17%). The two TMPRSS2 eQTL variants associated with increased expression exhibited much more disparate allele frequencies across world populations.
The researchers find TMPRSS2 is part of a mucus secretory network, highly upregulated by type 2 (T2) inflammation through the action of interleukin-13, and that the interferon response to respiratory viruses highly upregulates ACE2 expression. IL-13 and virus infection mediated effects on ACE2 expression were also observed at the protein level in the airway epithelium. The researchers used the Echo Revolve microscope to perform immunofluorescence experiments to confirm that the effects of IL-13 and viral infection-mediated ACE2 expression can also be observed at the protein level of the respiratory epithelium.
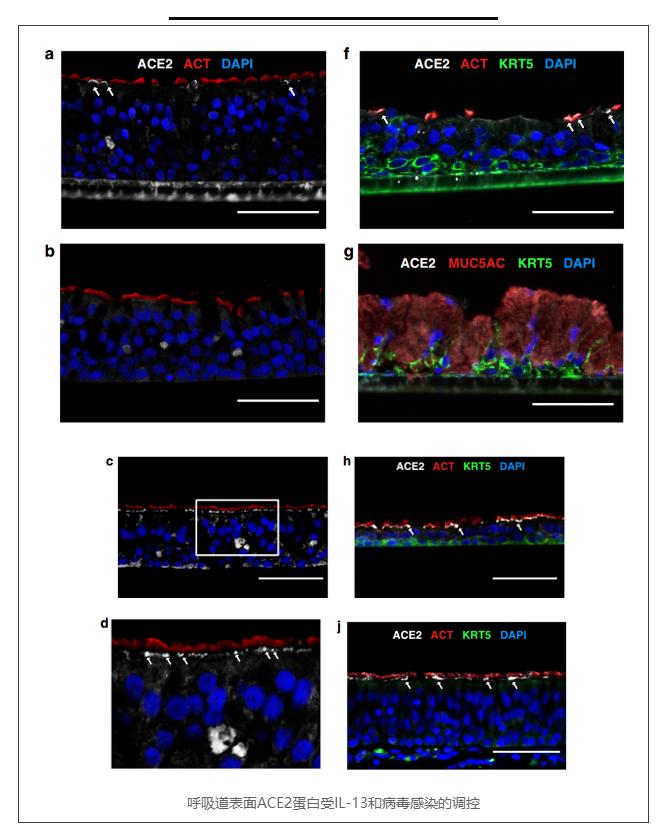
a–d Immunofluorescence staining of in vitro nasal airway epithelial ALI cultures (derived from a single GALA II asthmatic child) derived from vehicle-treated (a), 5 days IL-13(10 ng mL−1) treated (b), and 24 h HRV-A infected epithelium (c). Representative images of ciliated cells (ACT; red) and ACE2-positive (white) cells. The nuclei were counterstained with DAPI (blue). ACE2 protein was located in the apical compartment and decreased with IL-13 treatment and increased in HRV-A infection. Scale bars: 60 μm. d Zoomed in image of c.
f–h,i Immunofluorescence staining of in vitro nasal airway epithelial ALI cultures from a single child non-asthmatic donor derived from vehicle-treated (f), 21 days IL-13 (10 ng mL−1) treated (g), and 21 days DAPT(5 μM) treated epithelium (h). Representative images of ciliated cells (ACT; red), basal cells (KRT5; green), and ACE2-positive (white) cells. The nuclei were counterstained with DAPI (blue). ACE2 protein located in the apical compartment and decreased in IL-13 treatment and increased in DAPT treatment. Scale bars: 60 μm. j Immunofluorescence staining of in vivo trachea tissues from a single healthy donor. Representative images of ciliated cells (ACT; red), basal cells (KRT5; green), and ACE2-positive (white) cells. The nuclei were counterstained with DAPI (blue). ACE2 protein located in the apical compartment of ACT positive ciliated cells. Scale bars: 60 μm. All images were captured on the Echo Revolve R4 and images were cropped and resized using Affinity Designer.
Researchers finally confirmed the respiratory response of children to common coronavirus infections and found that these coronavirus infections produce host responses similar to other viral species, including upregulation of IL6 and ACE2. This research helps to further reveal the pathogenic mechanism of the new coronavirus and deepen our understanding of the new coronary pneumonia. We hope that we can analyze all the mechanisms of the disease caused by the new crown virus as soon as possible and defeat the new crown virus.
Echo Revolve Hybrid Microscope
Revolve has demonstrated its extraordinary flexibility. It can easily convert between upright and inverted microscopes. It innovatively combines upright and inverted microscopes into one, opening the hybrid era of microscopes.

☑ Retina display:Comparing eyepieces to human eyes.
☑ Full field observation: Clearer and more convenient.
☑ Multichannel fluorescence:Up to 4 EPI fluorescent channels,Multicolor fluorescence microscopic analysis can be done quickly and easily without darkroom.
☑ Automatic operation:The switch between the camera and the fluorescence channel is controlled by iPad Pro, which realizes fully automated operation.
☑ IOS-based Echo app:IOS-based Echo App is a professional microscope software developed in cooperation with the Apple team.
☑ Exquisite craftsmanship, high-end quality:Achieve extraordinary performance.
