

Stilla Technologies Digital PCR System
naica®Crystal Digital PCR System
NaicaTMCrystal Digital PCR System
Azure Biosystems Real-Time PCR Systems

Up to now, when screening for COVID-19 pneumonia, the nucleic acid test of nasopharyngeal swabs is mainly used because it is convenient and suitable for large-group testing. However, for some asymptomatic or mildly infected people, their condition recovers quickly after infection, and the COVID-19 may not be detected in 3 to 5 days. Studies have found that the duration of Postive-Nucleic Acid in stool or anal swabs of some infected people is longer than the duration of the upper respiratory tract. Therefore, increasing the nucleic acid detection of complex samples such as anal swabs can increase the detection rate of infected persons and reduce missed diagnosis.

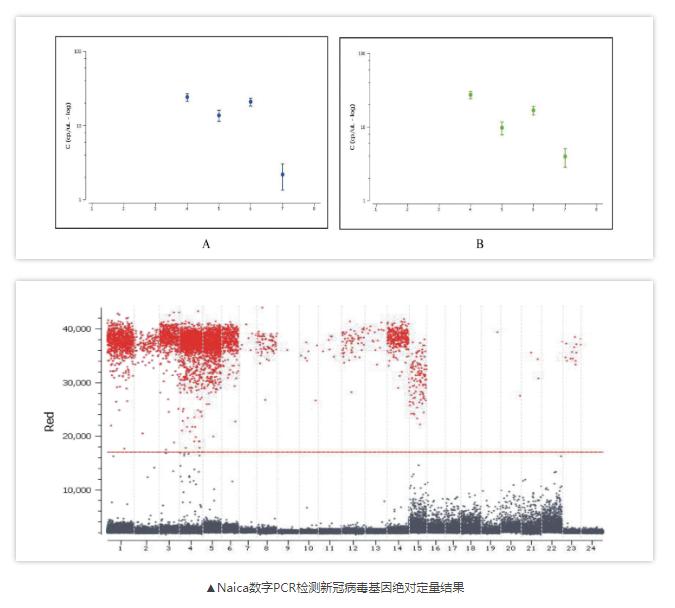
A few days ago, researchers from the Hunan Provincial Center for Disease Control and Prevention conducted simultaneous qPCR and Naica digital PCR (cd-PCR) nucleic acid tests on whole blood, urine, and stool samples from three confirmed COVID-19 patients at different times. It also compared the differences between the two methods for detecting 2019-nCoV in various samples, and proposed a comprehensive strategy to improve the nucleic acid detection of 2019-nCoV to provide more diagnostic support for patients with negative nucleic acid tests.
In their study, digital PCR can detect positive droplets in the early, middle, and late stage of cases, but qPCR cannot be detected in samples with onset time less than 5 days, and the positive nucleic acids detected are mainly in the middle and late stages. This also explains the limitation of the sensitivity of the detection method. At present, most of the false negative patients are in the early stage of disease development. In addition, in this experiment, digital PCR detected positive droplets in all three samples of severe cases, but because of the low viral load of blood, urine and suspicious stool samples, the qPCR test was negative.
Digital PCR technology can effectively overcome the problem of insufficient sensitivity of qPCR. It can capture blood, urine and suspicious stool or anal swab specimens with low viral load. It is a useful supplement to qPCR and helps clinicians accurately determine early-stage infection patients and judging whether the patient is really cured, it also has great significance for the long-term monitoring of 2019-nCoV.
Naica digital PCR technology has the advantages of high sensitivity, fully enclosed, and fast interpretation, and can be applied to the following cases:

1. Low-concentration sample detection, such as samples with ct value> 37 cycles, high-risk samples with ct value zero, and exclud false negative results ;
2. Accurate review of test samples;
3.Confirmation of patient discharge standards;
4. Regular follow-up testing of positive patients after discharge;
5.Complex sample detection of plasma, feces, urine, etc.;
6.Screening 2019-nCoV of samples from donation banks, sample banks, and blood banks;
7.Group and single testing, improve daily detection ability and high screening sensitivity.
