

Stilla Technologies Digital PCR System
naica®Crystal Digital PCR System
NaicaTMCrystal Digital PCR System
Azure Biosystems Real-Time PCR Systems

Paper -1:
The global outbreak of COVID-19is becoming increasingly serious. In addition to concerted efforts to combat the virus, researchers are also conducting more in-depth research on the COVID-19, such as vaccine development and whether the physiological functions of patients will be affected after infection. In particular, there has been much attention regarding the reproductive impact of COVID-19, and to this day, 27 viruses have been detected in semen and sperm. So in the context of the COVID-19, is SARS-CoV-2 (covid-19) present in semen? If very low viral load of SARS-CoV-2 (covid-19) is present in semen samples, then there must be a risk of transmission if it is used.
The Hunan Provincial Center for Disease Control and Prevention conducted a retrospective study using frozen semen collected by the Hunan Province Human Sperm Bank during and after the pandemic, and published it in the journal

Results:For individual blood and semen samples, the target genes, namely nucleocapsid protein (N) and open reading frame (ORF-1ab) genes, tested negative in all of the 100 paired samples. Further, as per cd-PCR results, there were >20,000 droplets per well in the RNA for each combined sample and no positive droplets were present for either of the aforementioned target genes. A total of 100 paired semen and blood samples from these two groups tested negative for SARS-CoV-2.
Conclusions:Cryopreserved semen at the Hunan Province Human Sperm Bank during and after the coronavirus disease 2019 (COVID-19) pandemic wave was free of SARS-CoV-2, and was judged safe for external use in the future.
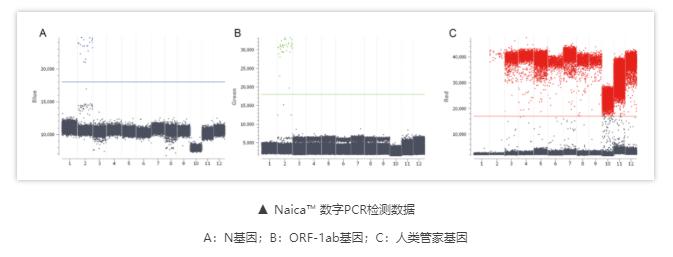
Paper -2:
This study performed qPCR and Naica™ digital PCR (cd-PCR) nucleic acid detection on three confirmed cases of novel coronavirus pneumonia based on whole blood, urine, and stool samples at different times, and compared the difference between the two methods to detect 2019-nCoV in various samples, a comprehensive strategy to improve the nucleic acid detection of 2019-nCoV is proposed to provide more diagnostic support for patients with negative nucleic acid tests.
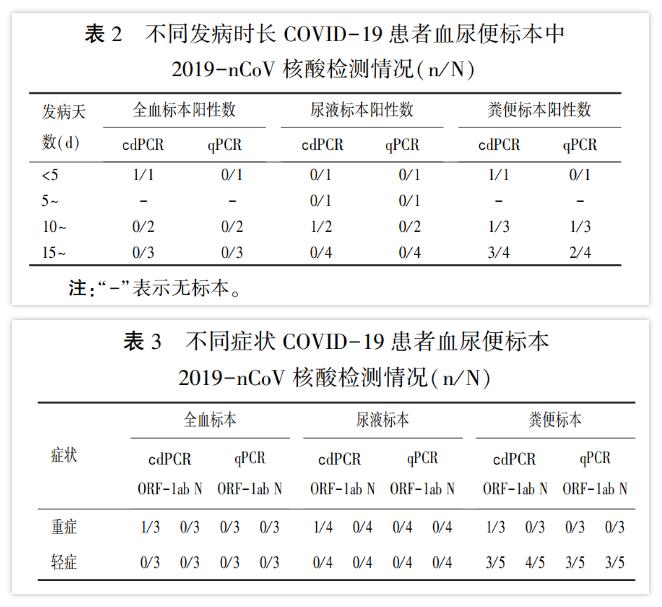
In this experiment, digital PCR can detect positive droplets in the early, middle and late onset specimens, while qPCR missed the specimens with onset less than 5 days, and the detected positive nucleic acids were mainly in the middle and late stages. (Table 2) This also explains the limitation of the sensitivity of the detection method. At present, most of the false negative patients are in the early stage of disease development. In addition, in this experiment, digital PCR detected positive droplets in all three specimens of severe cases. However, because of the low viral load of blood, urine, and suspicious stool specimens, the qPCR test was negative. (table 3)
In short, digital PCR technology can effectively overcome the problem of insufficient sensitivity of qPCR and accurately capture blood, urine and suspicious stool or anal swab specimens with low viral load.Digital PCR is a useful supplement to qPCR. It plays a positive role in helping clinicians to accurately determine early infection and whether the patient is truly cured, and is of great significance to the long-term monitoring of 2019-nCoV.
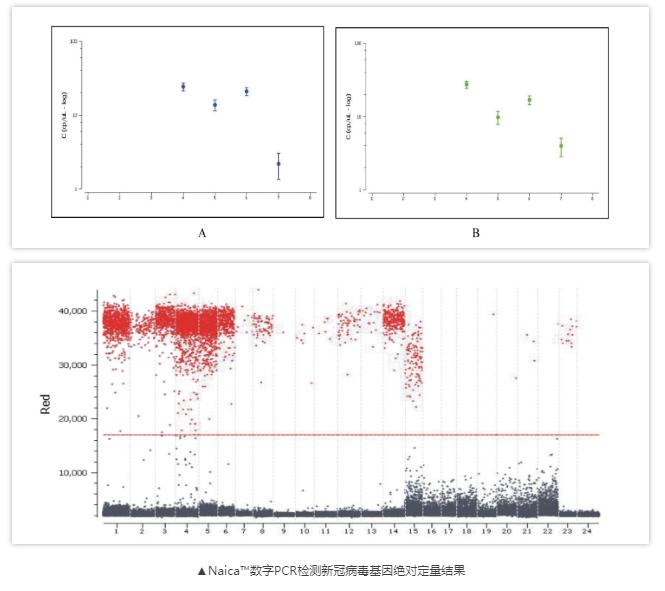
Both papers use the Naica™ digital PCR platform

Crystal Digital PCR™ is Stilla®’s next-generation technology for absolute quantification of nucleic acids. Using cutting-edge microfluidic innovations, this technology integrates the Digital PCR process in a single consumable.The sample is first flowed through a network of microchannels and partitioned into a large 2D array of 30,000 individual droplets, also called a droplet crystal, the whole process takes only 2.5 hours.With Crystal Digital PCR™, the combination of powerful image analysis and intuitive visual inspection offers an unmatched level of confidence in the digital PCR measurement, yielding data you can truly trust.
Operating procedures:

link:
Paper1:doi.org/10.1016/j.rbmo.2020.11.015
Paper2:doi:10.3969/j.issn.1006-3110.2020.11.009
