

Stilla Technologies Digital PCR System
naica®Crystal Digital PCR System
NaicaTMCrystal Digital PCR System
Azure Biosystems Real-Time PCR Systems


Cycloud Biotechnology's three channel high-sensitivity and fully-sealed digital PCR detection scheme for the novel coronavirus: Based on Naica crystal digital PCR system and three channel probe detection kit with high specificity, high sensitivity and fully sealed process to ensure safety.
Through the one-step reverse transcription-digital PCR-probe method, qualitative detection and absolute quantification of the novel coronavirus RNA with high specificity, high sensitivity, and fully sealed can be achieved by simply adding an RNA template, and reducing false negatives. The open reading frame 1ab (ORF1ab), nucleocapsid protein (N) gene region, and human endogenous quality control genes of the novel coronavirus were simultaneously detected in a sample tube.
Detection section: nucleoprotein (N) gene: FAM marker; open reading frame (ORF1ab) VIC marker; and internal standard gene: Cy5 marker to achieve quality control of sample size.
It is suitable for the re-examination of suspected samples of the novel coronavirus in the sample and qPCR test are negative (CT≥35).
Process of Test
Only one step of sample addition, manual operation takes only 5 minutes, 2.5 hours to obtain results

|
|
|
|
NaicaTM crystal digital PCR system |
2019-nCoV Detection Test Kit(For research use only. ) |
|
Three-channel fully closed nucleic acid absolute quantification tool |
Digital PCR one-step method Catlogue No: AK900103, Reaction: one set (24 tests) |
Naica crystal digital PCR system from France's Stilla Technologies, including Geode automated droplet generation and amplification system, Prism3 droplet collection and data analysis system. The single-layer droplet array is used to divide the PCR system into 25,000 to 30,000 uniform droplets. The target sequence is subjected to independent and stable PCR amplification under the same temperature cycling condition. By counting the positive droplets, Thus, three-channel nucleic acid copy number results were obtained based on the Poisson distribution statistics.
● Fully enclosed and automated workflow-safe and fast detection.
● Results can be obtained in 2.5 hours --- to improve work efficiency.
● Three-channel multiple detection --- for more information.
● Quality control and traceability of results-the results are credible and reliable.
The 2019-nCoV digital PCR detection kit (one-step digital PCR) can perform triple detection on one sample in a tube, including the nucleoprotein (N Gene): FAM labeling; open reading Open reading frame (ORF1ab) VIC marker; and internal standard gene: Cy5 marker, to achieve quality control of sample .
It is suitable for re-examination of suspected the novel coronavirus samples in samples and qPCR test are negative (CT≥35)
Features● The interpretation of results is simple and intuitive-directly output the number of virus copies. ● Higher sensitivity-for re-examination of suspicious samples. ● Strict quality control-Contains sample quality control genes and experimental quality control products. ● Unrestricted sample sources—strong tolerance to inhibitors. |
|
* Simultaneously developed digital PCR method to jointly detect the novel coronavirus / flu kit
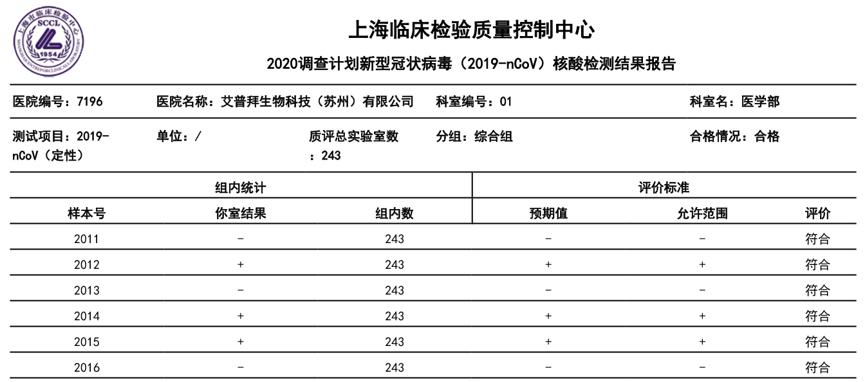
The kit has passed the novel coronavirus (2019-nCoV) nucleic acid detection capability verification test.
* For research use only.
